Many workers have pointed out that life on earth probably arose in the sea, and that the body water which is the environment of the cells, is similar to the ancient ocean from where life began. Although it appears quite tempting to draw comparison between environment of the cell and the ancient oceans but it would be rather an oversimplification in considering the cellular environment to be wholly fluid ignoring the presence of cells, fibres and ground substance.
Claude Bernarde (1949) first coined the term internal environment or milieu interieur for the state in the body in which the interstitial fluid that bathes the cells and the plasma, together maintain the normal morphology and function of the cells and tissues of the body. The mecha- nism by which the constancy of the internal environ- ment is maintained and ensured is called the homeostasis. For this purpose, living membranes with varying permeabilities such as vascular endothelium and the cell wall play important role in exchange of fluids, electrolytes, nutrients and metabolites across the compar- tments of body fluids.
The normal composition of internal environment consists of the following components :
1. WATER.
Water is the principal and essential consti- tuent of the body. The total body water in a normal adult male comprises 50-70% (average 60%) of the body weight and about 10% less in a normal adult female (average 50%). Thus, the body of a normal man weighing 65 kg contains approximately 40 litres of water The total body water (assuming average of 60%) is distributed into 2 main compartments of body fluids separated from each other by membranes freely permeable to water. These are as under :-
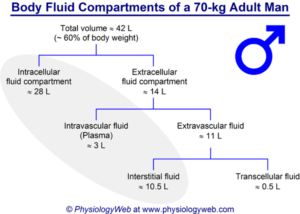
i) Intracellular fluid compartment. This comprises about 33% of the body weight, the bulk of which is contained in the muscles.
ii) Extracellular fluid compartment. This constitutes the remaining 27% of body weight containing water.
Included in this are the following 4 subdivisions of extracellular fluid (ECF):
a) Interstitial fluid including lymph fluid constitutes the major proportion of ECF (12% of body weight).
b) Intravascular fluid or blood plasma comprises about 5% of the body weight. Thus plasma content is about 3 litres of fluid out of 5 litres of total blood volume.
c) Mesenchymal tissues such as dense connective tissue, cartilage and bone contain body water that comprises about 9% of the body weight.
d) Transcellular fluid constitutes 1% of body weight. This is the fluid contained in the secretions of secretory cells of the body e.g. skin, salivary glands, mucous memb- ranes of alimentary and respiratory tracts, pancreas, liver and biliary tract, kidneys, gonads, thyroid, lacrimal gland and CSF.
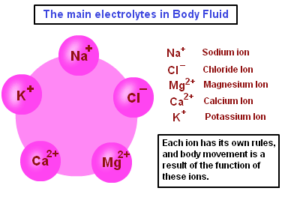
2. ELECTROLYTES. The concentration of cations (positively charged) and anions (negatively charged) is different in intracellular and extracellular fluids:
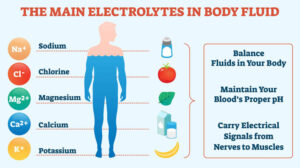
-In the intracellular fluid, the main cations are potassium and magnesium and the main anions are phosphates and proteins. It has low concentration of sodium and chloride.
-In the extracellular fluid, the predominant cation is sodium and the principal anions are chloride and bicarbo- nate. Besides these, a small proportion of non-diffusible proteins and some diffusible nutrients and metabolites such as glucose and urea are present in the ECF.
The essential difference between the two main sub- divisions of ECF is the higher protein content in the plasma than in the interstitial fluid which plays an important role in maintaining fluid balance.
The major functions of electrolytes are as follows:
i) Electrolytes are the main solutes in the body fluids for maintenance of acid-base equilibrium.
ii) Electrolytes maintain the proper osmolality and volume of body fluids (Osmolality is the solute concentration per kg water compared from osmolarity which is the solute concentration per litre solution).
iii) The concentration of certain electrolytes determines their specific physiologic functions e.g. the effect of calcium ions on neuromuscular excitability. The concen- tration of the major electrolytes is expressed in milli- equivalent (mEq) per litre so as to compare the values directly with each other.
NORMAL WATER AND ELECTROLYTE BALANCE (GIBBS-DONNAN EQUILIBRIUM) :
Normally, a state of balance exists between the amount of water absorbed into the body and that which is elimi- nated from the body. The water as well as electrolytes are distributed nearly constantly in different body fluid compartments.
1. Water is normally absorbed into the body from the bowel or is introduced parenterally; average intake being 2800 ml per day.
2. Water is eliminated from the body via:
-kidneys in the urine (average 1500 ml per day):
-via the skin as insensible loss in perspiration or as sweat (average 800 ml per day), though there is wide variation in loss via sweat depending upon weather,temperature, fever and exercise;
-via the lungs in exhaled air (average 400 ml per day); and
-minor losses via the faeces (average 100 ml per day) and lacrimal, nasal, oral, sexual and mammary (milk) secretions.
The cell wall as well as capillary endothelium are entirely permeable to water but they differ in their permeability to electrolytes. Capillary wall is completely permeable to electrolytes while the cell membrane is somewhat impermeable. As mentioned earlier, concen- tration of potassium and phosphate are high in the intra- cellular fluid whereas concentration of sodium and chloride are high in the ECF. The osmotic equilibrium between the two major body fluid compartments is maintained by the passage of water from or into the intracellular compartment. The 2 main subdivisions of ECF-blood plasma and interstitial fluid, are separated from each other by capillary wall which is freely perme- able to water but does not allow free passage of macro- molecules of plasma proteins resulting in higher protein content in the plasma.
ACID-BASE BALANCE :
Besides changes in the volume of fluids in the compart- ments, changes in ionic equilibrium affecting the acid- base balance of fluids occur. In terms of body fluids,
-an acid is a molecule or ion which is capable of giving off a hydrogen ion (H+ ion donor); and
-a base is a molecule or ion which is capable of taking up hydrogen ion (H” ion acceptor).
A number of acids such as carbonic, phosphoric, sulfuric, lactic, hydrochloric and ketoacids are formed during normal metabolic activity. However, carbonic acid is produced in largest amount as it is the end- product of aerobic tissue activity. In spite of these acids, the pH of the blood is kept constant at 7.4 +0.05 in health.
The pH of blood and acid-base balance are regulated in the body by the following factors:
1. BUFFER SYSTEM :
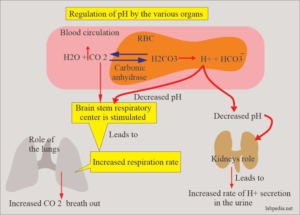
Buffers are substances which have weak acids and strong bases and limit the change in H+ ion concentration to the normal range. They are the first line of defense for maintaining acid-base balance and do so by taking up H ions when the pH rises. The most important buffer which regulates the pH of blood is bicarbonate-carbonic acid system followed by intracellular buffering action of haemoglobin and carbonic anhydrase in the red cells.
2. PULMONARY MECHANISM :
During respiration, CO, is removed by the lungs depending upon the partial pressure of CO, in the arterial blood. With ingestion of high quantity of acid-forming salts, ventilation is increased as seen in acidosis in diabetic ketosis and uraemia.
3. RENAL MECHANISM.
The other route by which H’ ions can be excreted from the body is in the urine. Here H’ ions secreted by the renal tubular cells are buffered in the glomerular filtrate by:
‘combining with phosphates to form phosphoric acid;
-combining with ammonia to form ammonium ions; and
-combining with filtered bicarbonate ions to form carbonic acid.
However, carbonic acid formed is dissociated to form CO, which diffuses back into the blood to reform bicarbonate ions.
PRESSURE GRADIENTS AND FLUID EXCHANGES :
Aside from water and electrolytes or crystalloids, both of which are freely interchanged between the interstitial fluid and plasma, the ECF contains colloids like proteins which minimally cross the capillary wall. These sub- stances exert pressures responsible for exchange between the interstitial fluid and plasma.
Normal Fluid Pressures :
1. OSMOTIC PRESSURE. This is the pressure exerted by the chemical constituents of the body fluids. Accordingly, osmotic pressure may be of the following types:
-Crystalloid osmotic pressure exerted by electrolytes present in the ECF and comprises the major portion of the total osmotic pressure.
– Colloid osmotic pressure or oncotic pressure exerted by proteins present in the ECF and constitutes a small part of the total osmotic pressure but is more significant physiologically. Since the protein content of the plasma is higher than that of interstitial fluid, oncotic pressure of plasma is higher (average 25 mmHg) than that of interstitial fluid (average 8 mmHg).
– Effective oncotic pressure is the difference between the higher oncotic pressure of plasma and the lower oncotic pressure of interstitial fluid and is the force that tends to draw fluid into the vessels.
2. HYDROSTATIC PRESSURE :
This is the capillary blood pressure.
There is considerable pressure gradient at the two ends of capillary loop-being higher at the arteriolar end (average 32 mmHg) than at the venular end (average 12 mmHg).
-Tissue tension is the hydrostatic pressure of interstitial fluid and is lower than the hydrostatic pressure in the capillary at either end (average. 4 mmHg).
– Effective hydrostatic pressure : is the difference between the higher hydrostatic pressure in the capillary and the lower tissue tension; it is the force that drives fluid through the capillary wall into the interstitial space.
Normal Fluid Exchanges :
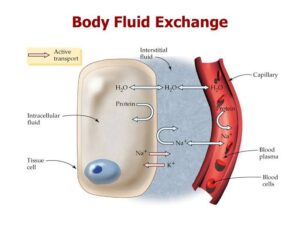
Normally, the fluid exchanges between the body compartments take place as under :
– At the arteriolar end of the capillary, the balance between the hydrostatic pressure (32 mmHg) and plasma oncotic pressure (25 mmHg) is the hydrostatic pressure of 7 mmHg which is the outward-driving force so that a small quantity of fluid and solutes leave the vessel to enter the interstitial space.
– At the venular end of the capillary, the balance between the hydrostatic pressure (12 mmHg) and plasma oncotic pressure (25 mmHg) is the oncotic pressure of 13 mmHg which is the inward-driving force so that the fluid and solutes re-enter the plasma.
– The tissue fluid left after exchanges across the capillary wall escapes into the lymphatics from where it is finally drained into venous circulation.
– Tissue factors i.e. oncotic pressure of interstitial fluid and tissue tension, are normally small and insignificant forces opposing the plasma hydrostatic pressure and capillary hydrostatic pressure, respectively.