Definition
Dental calculus is an adherent, calcified or calcifying mass that forms on the surfaces of teeth and dental appliances. It is covered on its external surface by vital, tightly adherent, nonmineralized plaque.
Types:
Depending upon the position of calculus in relation to the marginal gingiva it is classified as:
1. Supragingival calculus.
2. Subgingival calculus.
Supragingival Calculus
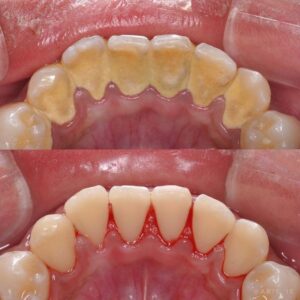
It is the tightly adherent calcified deposit that forms on the clinical crowns of the teeth above the free gingival margin. Hence it is clinically-visible. It is also called as salivary calculus because it forms from the saliva.
Subgingival Calculus
As the name implies, it is that calcified deposits that is formed on the root surfaces below the free marginal gingiva. It is believed to be formed from the gingival exudate and hence called serumal calculus .
Structure
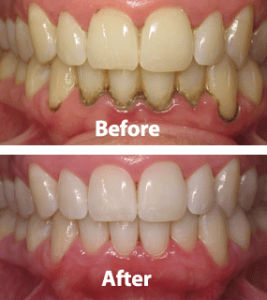
The deposits of supragingival calculus are usually whitish- yellow in color and can get stained by tobacco or food pigments, consistency is hard and clay-like. Since they derive the mineral salts from salivary secretions, they are most abundant on the lingual surfaces of lower anterior teeth, opposite Wharton’s duct and Bartholin’s duct and buccal aspects of maxillary molars opposite the Stenson’s duct .
Subgingival calculus is usually dark-brown or greenish-black in color and the deposits are firmly attached to the tooth surface. Since they are hard and firm, it cannot be removed easily. Unlike supragingival calculus, subgingival calculus can be found on any root surface with a periodontal pocket. Morphologically, it can appear in different forms, most commonly ring-like or ledge-like formations and crusty, spiny or nodular deposits. Less frequently it can be seen as finger-like and fern-like formations .
Composition :
It consists of inorganic and organic components .
Inorganic Components
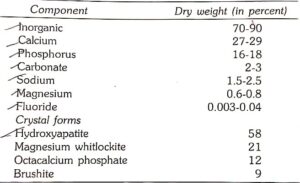
Trace amounts of zinc, strontium, bromine, copper, manganese, gold and aluminium are also seen. Atleast, two-thirds of inorganic component is crystalline in structure. The main crystal forms are: Hydroxyapatite. Magnesium whitlockite, Octacalcium phosphate and Brushite.
Organic Components

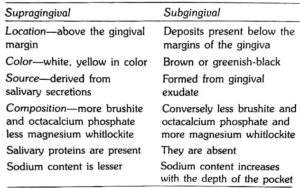
Differences between Supragingival and Subgingival Calculus :
Attachment to the Tooth Surface
Four types of attachment of calculus to tooth surface have been reported.
1. Attachment by means of an organic pellicle.
2. Mechanical interlocking into surface irregularities such as resorption lacunae and caries.
3. Penetration of calculus bacteria into cementum.
4. Close adaptation of calculus under surface depre- ssions to the gently sloping mounds of the unaltered cementum surface.
Calculus when embedded deeply in cementum may appear similar in morphology and thus has been termed as calculocementum.
Formation of Calculus :
Calculus is nothing but, dental plaque that has undergone mineralization. Calculus is formed by the precipitation of mineral salts, which can start between 1st and 14th day of plaque formation. In two days plaque can be 50 percent mineralized and 60 to 90 percent gets mineralized in 12 days. Calcification starts in separate foci on the inner surface of the plaque. These foci of mineralization gradually increase in size and coalesce to form a solid mass of calculus.
Calculus formation continues until it reaches maximum levels in about 10 weeks and 6 months, after which there is a decline in its formation, due to mechanical wear from food and from the lips, cheeks and tongue. This decline is referred to as reversal phenomenon.
Theories of Calculus Formation :
It can be explained mainly under two categories:
1. Precipitation of minerals can occur from a local rise in the degree of saturation of calcium and phosphate ions, this is explained in,
a. Booster mechanism: According to this theory, precipitation of calcium phosphate salts results from a local rise in the pH of the saliva. Factors such as loss of carbon dioxide and production of ammonia could lead to rise in pH.
Other ways by which the precipitation of
calcium phosphate salts can occur are:
b. Colloidal proteins in saliva bind to calcium and phosphate ions thus producing a super-saturated solution. When saliva stagnates in the oral cavity, colloids settle and result in the precipitation of calcium and phosphorous salts.
c. Phosphatase liberated from dental plaque,desquamated epithelial cells, or bacteria precipitate calcium phosphate by hydrolyzing organic phosphates in saliva, thus increasing the concentration of free phosphate ions.
2. Another concept that has been most widely held is “Epitactic Concept”. (heterogenous nucleation). According to this, seeding agents induce small foci of calcification. These foci enlarge and coalesce to form calculus. Hence more appropriately called as heterogenous nucleation. The seeding agents in calculus is not clearly known, but suspected agents could be, intercellular matrix of plaque, carbohydrate protein complexes and plaque bacteria.
3. Inhibition theory: This theory considers the possibility of calcification occurring only at specific sites because, there exists an inhibiting mechanism at non-calcifying sites. Where ever calcification occurs, the inhibitor is either altered or removed. One such inhibiting agents could be pyrophosphate which prevents the initial nucleus from growing, by possibly ‘poisoning’ the growth centers of the crystal.
Pathogenic Potential of Calculus in Periodontal Diseases :
Before 1960s the belief was that calculus was the principle etiologic factor in periodontal diseases. However, the current view is that the initial damage to the gingival margin in the periodontal disease is due to the pathogenic effects of microorganisms in plaque. However, the effect could get more pronounced by calculus accumulation because it further provides retention of more plaque microorganisms.
Hence, there is no doubt that, the mineralized deposits can-
a. Bring the bacterial deposits more closely to the supporting structures.
b. Interfere with the local self cleansing defense mechanisms.
c. And also enable the patients to perform proper oral hygiene methods.